Structural and dynamic properties of oxygen vacancies in perovskite oxides—analysis of defect chemistry by modern multi-frequency and pulsed EPR techniques
Abstract
Multi-frequency and pulsed electron paramagnetic resonance (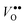 ) such that valency altered
) such that valency altered 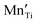 acceptor states or
acceptor states or 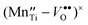 defect complexes are formed. Additionally, the trapping of charge carriers at the intrinsic ‘reduced’ B-site ions,
defect complexes are formed. Additionally, the trapping of charge carriers at the intrinsic ‘reduced’ B-site ions, 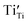 and
and 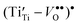 , can be observed by means of
, can be observed by means of


 Please wait while we load your content...
Please wait while we load your content...