Bond-energy decoupling: principle and application to heterogeneous catalysis†
Abstract
The chemical bond is a still mysterious force that glues atoms together to form molecules and materials. Unlike conventional electromagnetic interactions, the chemical bond cannot be quantified in strength by any known property of the participating species. Here we report an extraordinary principle that the bond energy (EAB), a quantitative measure of the bond strength, can be decoupled into two contributions of the participating reactants, with each described by a characteristic complex quantity (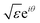 ):
): 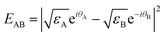 . Such a principle has been verified by more than 300 typical bonds, including covalent bonds in molecules and
. Such a principle has been verified by more than 300 typical bonds, including covalent bonds in molecules and


 Please wait while we load your content...
Please wait while we load your content...