The voltammetric determination of peroxynitrite at a mercury film electrode
Abstract
This report describes the direct voltammetric detection of peroxynitrite (PN, ONOO−), an important analyte in many physiological biochemical pathways, at a mercury film 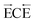 mechanism is proposed to explain this behaviour involving the initial formation of a thin film of Hg(I) oxide on the electrode which is then oxidised by PN to form HgO in a chemical step. The HgO is then reduced back to Hg(0) at the electrode potential of interest resulting in a net cathodic current. The parameters affecting the voltammetry of PN at a MFE, including the concentration of PN (10−5–10−4 M), the effect of varying scan rate (5–100 mV s−1) and pH are investigated and the kinetics of PN decay in alkaline solutions ranging from pH 9–13 are explored electrochemically.
mechanism is proposed to explain this behaviour involving the initial formation of a thin film of Hg(I) oxide on the electrode which is then oxidised by PN to form HgO in a chemical step. The HgO is then reduced back to Hg(0) at the electrode potential of interest resulting in a net cathodic current. The parameters affecting the voltammetry of PN at a MFE, including the concentration of PN (10−5–10−4 M), the effect of varying scan rate (5–100 mV s−1) and pH are investigated and the kinetics of PN decay in alkaline solutions ranging from pH 9–13 are explored electrochemically.


 Please wait while we load your content...
Please wait while we load your content...