Peroxy radical isomerization in the oxidation of isoprene†
Abstract
We report experimental evidence for the formation of C5-hydroperoxyaldehydes (HPALDs) from 1,6-H-shift isomerizations in 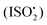 relative to the rate of reaction of
relative to the rate of reaction of 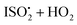 is
is 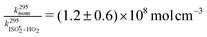 , or k295isom ≃ 0.002 s−1. The temperature dependence of this rate was determined through experiments conducted at 295, 310 and 318 K and is well described by
, or k295isom ≃ 0.002 s−1. The temperature dependence of this rate was determined through experiments conducted at 295, 310 and 318 K and is well described by 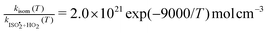 . The overall uncertainty in the isomerization rate (relative to
. The overall uncertainty in the isomerization rate (relative to 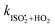 ) is estimated to be 50%.
) is estimated to be 50%.


 Please wait while we load your content...
Please wait while we load your content...