Improving electrochemical properties of hydrothermally synthesized LiFePO4 powders is of immense technological significance and has been a subject of much scientific inquiry for many years. As reported previously, reversing the feeding sequence of starting materials and/or introducing ethylene glycol (EG) could significantly improve the electrochemical performance of hydrothermally synthesized LiFePO4. However, the mechanism remains unclear. Here, we report a systematic study to understand the mechanism from viewpoints of crystal growth and defect concentration control. Combining the results of experimental and theoretical investigations, the improvement in electrochemical performance is attributed to simultaneous suppression of crystal growth along the [010] direction and reduced defect concentration of the 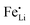 antisite. The reduction in antisite defects is readily monitored by significant red shift of the infrared (IR) absorption band around 1000 cm−1 which is assigned to the symmetric stretching P–O vibration of the PO4 tetrahedron, as indicated by theoretical calculation. With this knowledge in mind, an output as high as 450 g L−1 (autoclave volume), and an enhanced specific discharge capacity of 165 A h kg−1 (close to the theoretical unity of 170 A h kg−1) at 0.1 C are achieved.
antisite. The reduction in antisite defects is readily monitored by significant red shift of the infrared (IR) absorption band around 1000 cm−1 which is assigned to the symmetric stretching P–O vibration of the PO4 tetrahedron, as indicated by theoretical calculation. With this knowledge in mind, an output as high as 450 g L−1 (autoclave volume), and an enhanced specific discharge capacity of 165 A h kg−1 (close to the theoretical unity of 170 A h kg−1) at 0.1 C are achieved.
![Graphical abstract: Hydrothermally synthesized LiFePO4 crystals with enhanced electrochemical properties: simultaneous suppression of crystal growth along [010] and antisite defect formation](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CP23433E&imageInfo.ImageIdentifier.Year=2012)
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
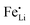 antisite. The
antisite. The ![Graphical abstract: Hydrothermally synthesized LiFePO4 crystals with enhanced electrochemical properties: simultaneous suppression of crystal growth along [010] and antisite defect formation](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CP23433E&imageInfo.ImageIdentifier.Year=2012)

 Please wait while we load your content...
Please wait while we load your content...