This manuscript explores the product distribution of the reaction of carbon dioxide with reactive iron(I) complexes supported by tris(phosphino)borate ligands, [PhBPR3]− ([PhBPR3]− = [PhB(CH2PR2)3]−; R = CH2Cy, Ph, iPr, mter; mter = 3,5-meta-terphenyl). Our studies reveal an interesting and unexpected role for the solvent medium with respect to the course of the CO2 activation reaction. For instance, exposure of methylcyclohexane (MeCy) solutions of 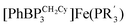 to CO2 yields the partial decarbonylation product
to CO2 yields the partial decarbonylation product 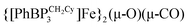 . When the reaction is instead carried out in benzene or THF, reductive coupling of CO2 occurs to give the bridging oxalate species
. When the reaction is instead carried out in benzene or THF, reductive coupling of CO2 occurs to give the bridging oxalate species 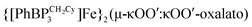 . Reaction studies aimed at understanding this solvent effect are presented, and suggest that the product profile is ultimately determined by the ability of the solvent to coordinate the iron center. When more sterically encumbering auxiliary ligands are employed to support the iron(I) center (i.e., [PhBPPh3]− and [PhBPiPr3]−), complete decarbonylation is observed to afford structurally unusual diiron(II) products of the type {[PhBPR3]Fe}2(μ-O). A mechanistic hypothesis that is consistent with the collection of results described is offered, and suggests that reductive coupling of CO2 likely occurs from an electronically saturated “FeII–CO2˙−” species.
. Reaction studies aimed at understanding this solvent effect are presented, and suggest that the product profile is ultimately determined by the ability of the solvent to coordinate the iron center. When more sterically encumbering auxiliary ligands are employed to support the iron(I) center (i.e., [PhBPPh3]− and [PhBPiPr3]−), complete decarbonylation is observed to afford structurally unusual diiron(II) products of the type {[PhBPR3]Fe}2(μ-O). A mechanistic hypothesis that is consistent with the collection of results described is offered, and suggests that reductive coupling of CO2 likely occurs from an electronically saturated “FeII–CO2˙−” species.

You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
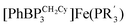 to CO2 yields the partial decarbonylation product
to CO2 yields the partial decarbonylation product 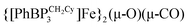 . When the reaction is instead carried out in
. When the reaction is instead carried out in 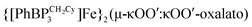 . Reaction studies aimed at understanding this
. Reaction studies aimed at understanding this 

 Please wait while we load your content...
Please wait while we load your content...