High-throughput screening of small-molecule adsorption in MOF†
Abstract
Using high-throughput screening coupled with state-of-the-art van der Waals density functional theory, we investigate the adsorption properties of four important molecules, H2, CO2, CH4, and H2O in MOF-74-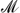 with
with 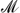 = Be, Mg, Al, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Sr, Zr, Nb, Ru, Rh, Pd, La, W, Os, Ir, and Pt. We show that high-throughput techniques can aid in speeding up the development and refinement of effective materials for hydrogen storage, carbon capture, and gas separation. The exploration of the configurational adsorption space allows us to extract crucial information concerning, for example, the competition of water with CO2 for the adsorption binding sites. We find that only a few noble metals—Rh, Pd, Os, Ir, and Pt—favor the adsorption of CO2 and hence are potential candidates for effective carbon-capture materials. Our findings further reveal significant differences in the binding characteristics of H2, CO2, CH4, and H2O within the MOF structure, indicating that molecular blends can be successfully separated by these nano-porous materials.
= Be, Mg, Al, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Sr, Zr, Nb, Ru, Rh, Pd, La, W, Os, Ir, and Pt. We show that high-throughput techniques can aid in speeding up the development and refinement of effective materials for hydrogen storage, carbon capture, and gas separation. The exploration of the configurational adsorption space allows us to extract crucial information concerning, for example, the competition of water with CO2 for the adsorption binding sites. We find that only a few noble metals—Rh, Pd, Os, Ir, and Pt—favor the adsorption of CO2 and hence are potential candidates for effective carbon-capture materials. Our findings further reveal significant differences in the binding characteristics of H2, CO2, CH4, and H2O within the MOF structure, indicating that molecular blends can be successfully separated by these nano-porous materials.


 Please wait while we load your content...
Please wait while we load your content...