Carbon monoxide protonation in condensed phases and bonding to surface superacidic Brønsted centers†
Abstract
Using infrared (IR) spectroscopy and density functional theory (DFT) calculations, interaction of CO with the strongest known pure Brønsted carborane superacids, H(CHB11Hal11) (Hal = F, Cl), was studied. CO readily interacted at room temperature with H(CHB11F11) acid, forming a mixture of bulk salts of formyl and isoformyl cations, which were in equilibrium An−⋯H+CO 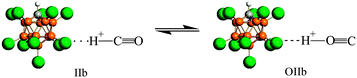 COH+⋯An−. The bonding of CO to the surface Brønsted centers of the weaker acid, H(CHB11Cl11), resulted in breaking of the bridged H-bonds of the acid polymers without proton transfer (PT) to CO. The binding occurred via the C atom (blue shift ΔνCO up to +155–167 cm−1, without PT) or via O atom (red shift ΔνCO up to −110 cm−1, without PT) always simultaneously, regardless of whether H+ is transferred to CO. IR spectra of all species were interpreted by B3LYP/cc-pVQZ calculations of the simple models, which adequately mimic the ability of carborane acids to form L⋯H+CO, LH+⋯CO, COH+⋯L, and CO⋯H+L compounds (L = bases). The CO bond in all compounds was triple. Acidic strength of the Brønsted centers of commonly used acid catalysts, even so-called superacidic catalysts, is not sufficient for the formation of the compounds studied.
COH+⋯An−. The bonding of CO to the surface Brønsted centers of the weaker acid, H(CHB11Cl11), resulted in breaking of the bridged H-bonds of the acid polymers without proton transfer (PT) to CO. The binding occurred via the C atom (blue shift ΔνCO up to +155–167 cm−1, without PT) or via O atom (red shift ΔνCO up to −110 cm−1, without PT) always simultaneously, regardless of whether H+ is transferred to CO. IR spectra of all species were interpreted by B3LYP/cc-pVQZ calculations of the simple models, which adequately mimic the ability of carborane acids to form L⋯H+CO, LH+⋯CO, COH+⋯L, and CO⋯H+L compounds (L = bases). The CO bond in all compounds was triple. Acidic strength of the Brønsted centers of commonly used acid catalysts, even so-called superacidic catalysts, is not sufficient for the formation of the compounds studied.


 Please wait while we load your content...
Please wait while we load your content...