Steric effect of the dithiolato linker on the reduction mechanism of [Fe2(CO)6{μ-(XCH2)2CRR′}] hydrogenase models (X = S, Se)†
Abstract
Studying the redox features of the [FeFe]-hydrogenase models is essential for understanding the function of the H cluster. The reduction of the [FeFe]-hydrogenase models of the type [Fe2(CO)6{μ-(XCH2)2E}] (X = S, Se) is described to occur either via sequential transfer of two electrons at 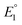 and
and 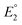 for the first and the second reduction steps, respectively, where
for the first and the second reduction steps, respectively, where 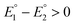 , or via transfer of two electrons at the same applied potential due to potential inversion of the two reduction steps, i.e.
, or via transfer of two electrons at the same applied potential due to potential inversion of the two reduction steps, i.e.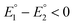 . Typically, the phenomenon of potential inversion is observed when a structural change intervenes in the cathodic process stabilizing the reduced species. In this report, we investigate the mechanism of the cathodic process of series of models [Fe2(CO)6{μ-(XCH2)2E}] (X = S or Se and E = CH2, CHMe or CMe2) applying cyclic voltammetry. The studies herein show the remarkable influence of the steric bulk of E toward the cathodic process, such that only complexes with E = CMe2 are reduced with inverted potentials due to occurrence of an ECE mechanism (E = electrochemical process, C = chemical process) of reduction. Moreover, we describe the catalytic behaviour of these models toward reduction of protons using acetic acid, AcOH, as a proton source.
. Typically, the phenomenon of potential inversion is observed when a structural change intervenes in the cathodic process stabilizing the reduced species. In this report, we investigate the mechanism of the cathodic process of series of models [Fe2(CO)6{μ-(XCH2)2E}] (X = S or Se and E = CH2, CHMe or CMe2) applying cyclic voltammetry. The studies herein show the remarkable influence of the steric bulk of E toward the cathodic process, such that only complexes with E = CMe2 are reduced with inverted potentials due to occurrence of an ECE mechanism (E = electrochemical process, C = chemical process) of reduction. Moreover, we describe the catalytic behaviour of these models toward reduction of protons using acetic acid, AcOH, as a proton source.
![Graphical abstract: Steric effect of the dithiolato linker on the reduction mechanism of [Fe2(CO)6{μ-(XCH2)2CRR′}] hydrogenase models (X = S, Se)](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5DT01387A&imageInfo.ImageIdentifier.Year=2015)

 Please wait while we load your content...
Please wait while we load your content...