On the constitution and thermodynamic modelling of the system Ti–Ni–Sn†
Abstract
Phase equilibria of the system Ti–Ni–Sn have been determined for the isothermal section at 950 °C based on X-ray powder diffraction (XPD) and electron probe microanalysis (EPMA) of about 60 ternary alloys in as cast and annealed state. The section is characterized by the formation of four ternary compounds labelled τ1 to τ4. Whereas two of the ternary compounds are found without significant homogeneity regions: τ1-TiNiSn (half Heusler phase, MgAgAs-type), τ3-Ti2Ni2Sn (U2Pt2Sn-type), τ4-(Ti1−x−ySnxNiy)Ni3 with AuCu3-type exhibits a solution range (0.22 ≤ x ≤ 0.66 and 0.22 ≥ y ≥ 0.02) and a particularly large homogeneity region is recorded for τ2-Ti1+yNi2−xSn1−y (Heusler phase, MnCu2Al-type). Extended solid solutions starting from binary phases at 950 °C have been evaluated for Ti5Ni1−xSn3 (filled Mn5Si3 = Ti5Ga4-type; 0 ≤ x ≤ 1), Ti1−xSnxNi3 (TiNi3-type; 0 ≤ x ≤ 0.27) and (Ti1−xNix)1−ySny (CsCl-type) reaching a maximum solubility at x = 0.53, y = 0.06). From differential thermal analysis (DTA) in alumina crucibles under argon a complete liquidus surface has been elucidated revealing congruent melting for τ2-TiNi2Sn at 1447 °C, but incongruent melting for τ1-TiNiSn (pseudobinary peritectic formation: 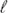 + τ2 ↔ τ1 at 1180 °C), τ3-Ti2Ni2Sn (peritectic formation: L + τ2 + Ti5NiSn3 ↔ τ3 at 1151 °C) and τ4-Ti1−xSnxNi3 (peritectic formation: L + TiNi3 + (Ni) ↔ τ4 at 1157 °C). A Schultz–Scheil diagram for the solidification behavior was constructed for the entire diagram involving 20 isothermal four-phase reactions in the ternary. For a thermodynamic CALPHAD assessment of the ternary diagram we relied on the binary boundary systems as modelled in the literature. As thermodynamic data in the ternary system were only available in the literature for the compounds TiNi2Sn and TiNiSn, heat of formation data were supplied by our density functional theory (DFT) calculations for Ti2Ni2Sn, as well as for the solid solutions, which were modelled for Ti1−xSnxNi3, Ti5Ni1−xSn3 and (Ti1−xNix)1−ySny. Thermodynamic calculation was performed with the Pandat software and finally showed a reasonably good agreement for all the 20 invariant reaction isotherms involving the liquid.
+ τ2 ↔ τ1 at 1180 °C), τ3-Ti2Ni2Sn (peritectic formation: L + τ2 + Ti5NiSn3 ↔ τ3 at 1151 °C) and τ4-Ti1−xSnxNi3 (peritectic formation: L + TiNi3 + (Ni) ↔ τ4 at 1157 °C). A Schultz–Scheil diagram for the solidification behavior was constructed for the entire diagram involving 20 isothermal four-phase reactions in the ternary. For a thermodynamic CALPHAD assessment of the ternary diagram we relied on the binary boundary systems as modelled in the literature. As thermodynamic data in the ternary system were only available in the literature for the compounds TiNi2Sn and TiNiSn, heat of formation data were supplied by our density functional theory (DFT) calculations for Ti2Ni2Sn, as well as for the solid solutions, which were modelled for Ti1−xSnxNi3, Ti5Ni1−xSn3 and (Ti1−xNix)1−ySny. Thermodynamic calculation was performed with the Pandat software and finally showed a reasonably good agreement for all the 20 invariant reaction isotherms involving the liquid.


 Please wait while we load your content...
Please wait while we load your content...