A van der Waals density functional investigation of carboranethiol self-assembled monolayers on Au(111)
Abstract
Isolated and full monolayer adsorption of various carboranethiol (C2B10H12S) isomers on the gold(111) surface has been investigated using both the standard and van der Waals density functional theory calculations. The effect of different molecular dipole moment orientations on the low energy adlayer geometries, the binding characteristics and the electronic properties of the self-assembled monolayers of these isomers has been studied. Specifically, the binding energy and work function changes associated with different molecules show a correlation with their dipole moments. The adsorption is favored for the isomers with dipole moments parallel to the surface. Of the two possible unit cell structures, (5 × 5) was found to be more stable than 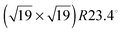 .
.


 Please wait while we load your content...
Please wait while we load your content...