Formation and migration of hydride ions in BaTiO3−xHx oxyhydride
Abstract
Oxyhydrides have recently emerged as a class of novel ionic conductors with potential application as, for instance, electrolytes in all-solid-state batteries. Little is, however, known about the hydride ion migration mechanism. In this contribution, we address with first principles calculations the formation and stability of hydride defects in BaTiO3 with emphasis on the hydride ion transport mechanism. Our results demonstrate that hydride ions are solely stabilized at vacant oxygen sites in the BaTiO3 structure as 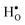 . These hydride ions induce electronic defects in the form of small polarons at adjacent Ti atoms, rationalizing the experimentally observed semi-conducting behavior and blue color of BaTiO3−xHx. Migration of hydride ion occurs in an oxygen-vacancy mediated mechanism, characterized by a barrier of 0.28 eV. Long range hydride ion transport is, however, limited by the low oxygen vacancy mobility and concentration. This mechanism describing hydride ion transport could be extended to other hydride ion conducting materials and sheds light on novel hydride ion conductors.
. These hydride ions induce electronic defects in the form of small polarons at adjacent Ti atoms, rationalizing the experimentally observed semi-conducting behavior and blue color of BaTiO3−xHx. Migration of hydride ion occurs in an oxygen-vacancy mediated mechanism, characterized by a barrier of 0.28 eV. Long range hydride ion transport is, however, limited by the low oxygen vacancy mobility and concentration. This mechanism describing hydride ion transport could be extended to other hydride ion conducting materials and sheds light on novel hydride ion conductors.


 Please wait while we load your content...
Please wait while we load your content...