Spin-reversal energy barriers of 305 K for Fe2+ d6 ions with linear ligand coordination
Abstract
A remarkably large magnetic anisotropy energy of 305 K is computed by quantum chemistry methods for divalent Fe2+ d6 substitutes at Li-ion sites with D6h point-group symmetry within the solid-state matrix of Li3N. This is similar to values calculated by the same approach and confirmed experimentally for linearly coordinated monovalent Fe1+ d7 species, among the largest so far in the research area of single-molecule magnets. Our ab initio results therefore mark a new exciting exploration path in the search for superior single-molecule magnets, rooted in the 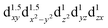 configuration of d6 transition-metal ions with linear or quasilinear nearest-neighbor coordination. This d6 axial anisotropy may be kept robust even for symmetries lower than D6h, provided the ligand and farther-neighbor environment is engineered such that the
configuration of d6 transition-metal ions with linear or quasilinear nearest-neighbor coordination. This d6 axial anisotropy may be kept robust even for symmetries lower than D6h, provided the ligand and farther-neighbor environment is engineered such that the 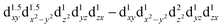 splitting remains large enough.
splitting remains large enough.



 Please wait while we load your content...
Please wait while we load your content...