Solubility temperature and solvent dependence and preferential solvation of citrus flavonoid naringin in aqueous DMSO mixtures: an experimental and molecular dynamics simulation study
Abstract
This study describes the thermodynamics of dissolution of flavonoid naringin in different aqueous solutions of dimethyl sulfoxide (DMSO) containing 0–100% (w/w) under atmospheric pressure and over a temperature range of 298.15 to 325.15 K. The temperature dependence of solubility of naringin was analyzed using the modified Apelblat equation model, ideal model, and the λH equation model. In a mean harmonic temperature, the dissolution thermodynamic parameters of naringin containing 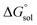 ,
, 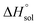 and
and 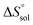 were also calculated. Furthermore, the effects of solvent composition on the solubility of this flavonoid were analyzed in terms of Hildebrand's solubility parameter (δH) and Kamlet, Abboud and Taft (KAT) solvatochromic parameters (α, β, and π*). Finally, the preferential solvation parameters of the flavonoid naringin by DMSO (δxDMSO,S) were determined from experimental solubility data using the inverse Kirkwood–Buff integrals (IKBIs). It was found that water preferentially solvates naringin in water-rich mixtures while DMSO forms local solvation shells in compositions from 50% (w/w) or xDMSO = 0.19 up to pure co-solvent. Moreover, the structure of solvation shells of naringin in the under study mixtures was obtained by molecular dynamics (MD) simulations. The computational results showed that in the compositions xDMSO > 0.20, the probability of presence of the DMSO molecules in vicinity of naringin is more than water molecules. These findings are compatible with the available IKBI data.
were also calculated. Furthermore, the effects of solvent composition on the solubility of this flavonoid were analyzed in terms of Hildebrand's solubility parameter (δH) and Kamlet, Abboud and Taft (KAT) solvatochromic parameters (α, β, and π*). Finally, the preferential solvation parameters of the flavonoid naringin by DMSO (δxDMSO,S) were determined from experimental solubility data using the inverse Kirkwood–Buff integrals (IKBIs). It was found that water preferentially solvates naringin in water-rich mixtures while DMSO forms local solvation shells in compositions from 50% (w/w) or xDMSO = 0.19 up to pure co-solvent. Moreover, the structure of solvation shells of naringin in the under study mixtures was obtained by molecular dynamics (MD) simulations. The computational results showed that in the compositions xDMSO > 0.20, the probability of presence of the DMSO molecules in vicinity of naringin is more than water molecules. These findings are compatible with the available IKBI data.



 Please wait while we load your content...
Please wait while we load your content...