Solution structure of a sweet protein single-chain monellin determined by nuclear magnetic resonance and dynamical simulated annealing calculations.
Lee, S.Y., Lee, J.H., Chang, H.J., Cho, J.M., Jung, J.W., Lee, W.(1999) Biochemistry 38: 2340-2346
- PubMed: 10029527
- DOI: https://doi.org/10.1021/bi9822731
- Primary Citation of Related Structures:
1MNL - PubMed Abstract:
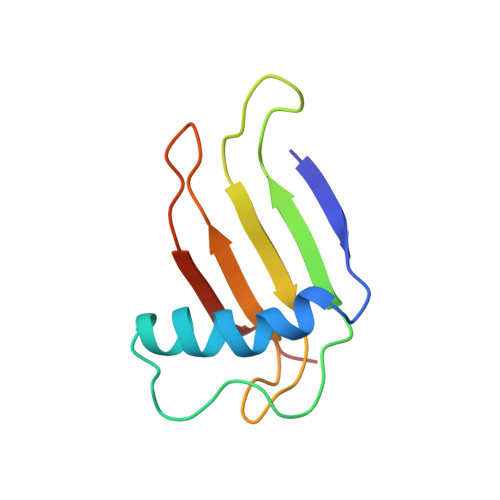
Single-chain monellin (SCM), which is an engineered 94-residue polypeptide, has proven to be as sweet as native two-chain monellin. SCM is more stable than the native monellin for both heat and acidic environments. Data from gel filtration HPLC and NMR indicate that the SCM exists as a monomer in aqueous solution. The solution structure of SCM has been determined by nuclear magnetic resonance (NMR) spectroscopy and dynamical simulated annealing calculations. A stable alpha-helix spanning residues Phe11-Ile26 and an antiparallel beta-sheet formed by residues 2-5, 36-38, 41-47, 54-64, 69-75, and 83-88 have been identified. The sheet was well defined by backbone-backbone NOEs, and the corresponding beta-strands were further confirmed by hydrogen bond networks based on amide hydrogen exchange data. Strands beta2 and beta3 are connected by a small bulge comprising residues Ile38-Cys41. A total of 993 distance and 56 dihedral angle restraints were used for simulated annealing calculations. The final simulated annealing structures (
k) converged well with a root-mean-square deviation (rmsd) between backbone atoms of 0.49 A for secondary structural regions and 0.70 A for backbone atoms excluding two loop regions. The average restraint energy-minimized (REM) structure exhibited root-mean-square deviations of 1.19 A for backbone atoms and 0.85 A for backbone atoms excluding two loop regions with respect to 20 k structures. The solution structure of SCM revealed that the long alpha-helix was folded into the concave side of a six-stranded antiparallel beta-sheet. The side chains of Tyr63 and Asp66 which are common to all sweet peptides showed an opposite orientation relative to H1 helix, and they were all solvent-exposed. Residues at the proposed dimeric interface in the X-ray structure were observed to be mostly solvent-exposed and demonstrated high degrees of flexibility.
Organizational Affiliation:
Department of Biochemistry, College of Science, Yonsei University, Seoul, Korea.