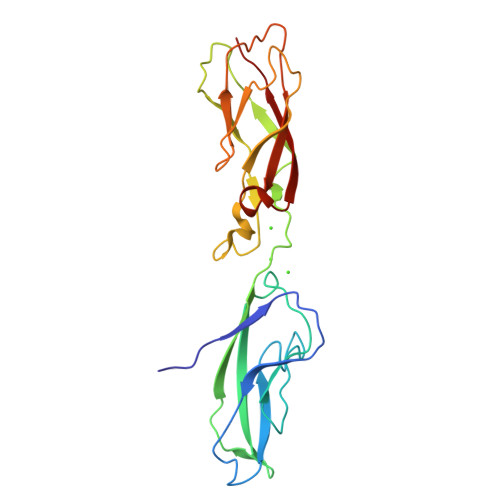
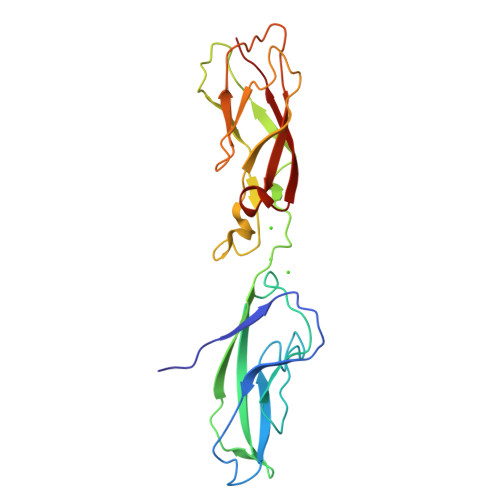
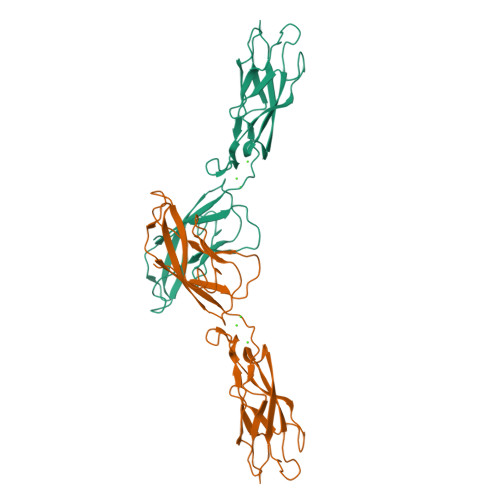
Proteolytic E-cadherin activation followed by solution NMR and X-ray crystallography.
Haussinger, D., Ahrens, T., Aberle, T., Engel, J., Stetefeld, J., Grzesiek, S.(2004) EMBO J 23: 1699-1708
- PubMed: 15071499
- DOI: https://doi.org/10.1038/sj.emboj.7600192
- Primary Citation of Related Structures:
1Q1P - PubMed Abstract:
Cellular adhesion by classical cadherins depends critically on the exact proteolytic removal of their N-terminal prosequences. In this combined solution NMR and X-ray crystallographic study, the consequences of propeptide cleavage of an epithelial cadherin construct (domains 1 and 2) were followed at atomic level. At low protein concentration, the N-terminal processing induces docking of the tryptophan-2 side-chain into a binding pocket on the same molecule. At high concentration, cleavage induces dimerization (KD=0.72 mM, k(off)=0.7 s(-1)) and concomitant intermolecular exchange of the betaA-strands and the tryptophan-2 side-chains. Thus, the cleavage represents the switch from a nonadhesive to the functional form of cadherin.
Organizational Affiliation:
Division of Structural Biology, Biozentrum, University of Basel, Basel, Switzerland.