Crystal structures of two hemoglobin components from the midge larva Propsilocerus akamusi (Orthocladiinae, Diptera).
Kuwada, T., Hasegawa, T., Sato, S., Sato, I., Ishikawa, K., Takagi, T., Shishikura, F.(2007) Gene 398: 29-34
- PubMed: 17590288
- DOI: https://doi.org/10.1016/j.gene.2007.02.049
- Primary Citation of Related Structures:
1X3K, 1X46 - PubMed Abstract:
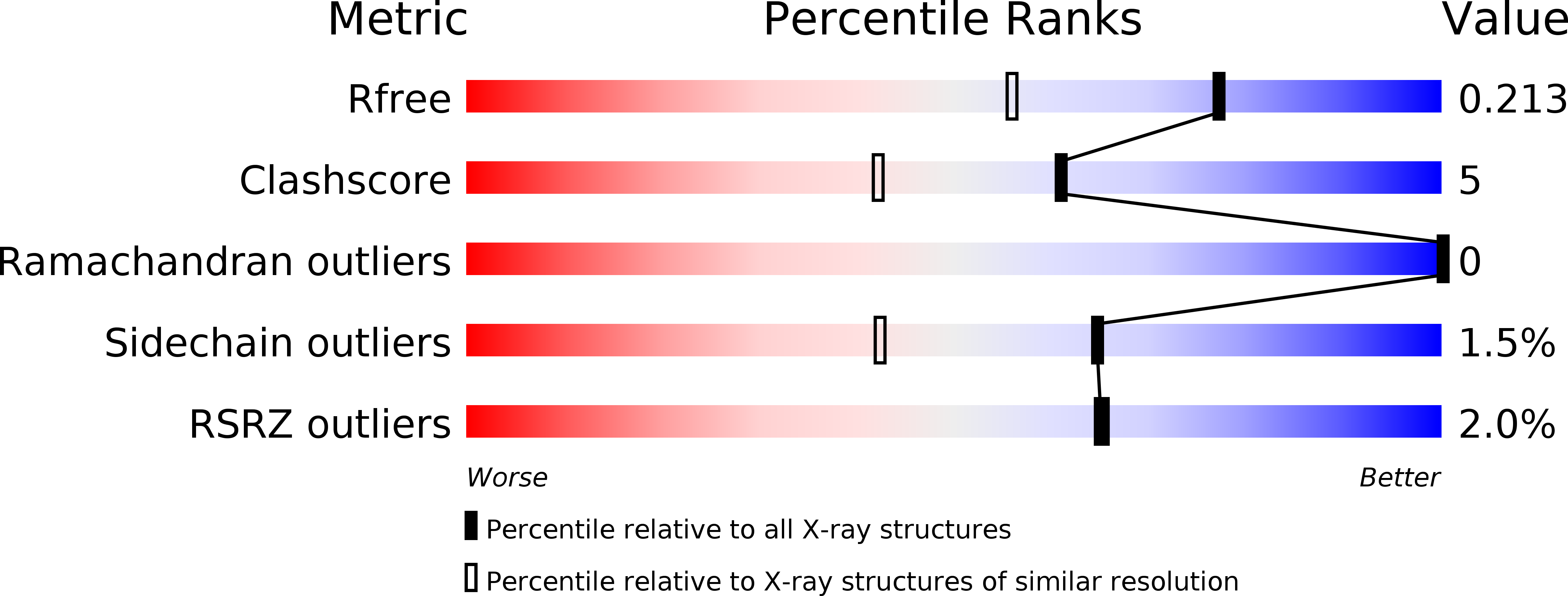
The polymorphic components of hemoglobin (Hb) of the midge larva Propsilocerus akamusi were classified into two distinct types dependent on their spectroscopic properties, normal absorption (N) and low absorption (L). Analyses of the amino acid sequences of component VII (N-type Hb) and component V (L-type Hb) from P. akamusi indicated that one remarkable difference is the replacement of the distal histidine (His) with isoleucine (Ile) in component V. To clarify the structural differences between the two Hb components, we determined the crystal structures of components V and VII at resolutions of 1.64 A and 1.50 A, respectively. These crystal structures indicated a short additional helix comprising three amino acid residues at the C-terminal region in component V, and a typical globin fold including eight helices in component VII. Comparison of the heme regions of the Hb components suggests that the structural changes of the heme region in component V on ligation differ from that of usual Hb.
Organizational Affiliation:
Advanced Research Institute for the Sciences and Humanities, Nihon University, Chiyoda-ku, Tokyo 102-8251, Japan.