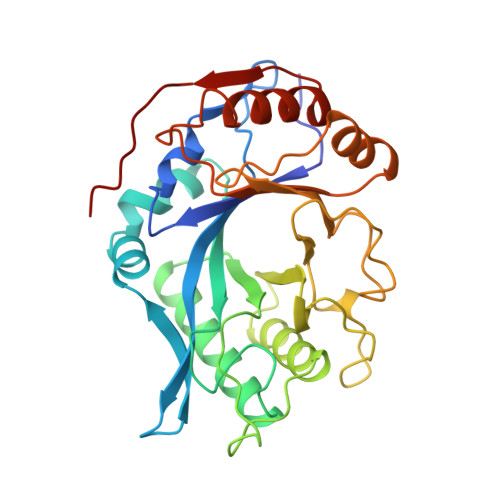
Crystal structure of endo-beta-N-acetylglucosaminidase F1, an alpha/beta-barrel enzyme adapted for a complex substrate.
Van Roey, P., Rao, V., Plummer Jr., T.H., Tarentino, A.L.(1994) Biochemistry 33: 13989-13996
- PubMed: 7947807
- DOI: https://doi.org/10.1021/bi00251a005
- Primary Citation of Related Structures:
2EBN - PubMed Abstract:
Endo-beta-N-acetylglucosaminidase F1 (Endo F1) is an endoglycosidase, secreted by Flavobacterium meningosepticum, that cleaves asparagine-linked oligosaccharides after the first N-acetylglucosamine residue. The enzyme is selective for high-mannose oligosaccharide chains. The crystal structure of Endo F1 has been determined at 2.0-A resolution. The molecular fold consists of a highly irregular alpha/beta-barrel, a commonly observed motif consisting of a cyclic 8-fold repeat of beta-strand/loop/alpha-helix units with an eight-stranded parallel beta-barrel at the center. Endo F1 lacks two of the alpha-helices, those of units 5 and 6. Instead, the links after beta-strands 5 and 6 consist of a short turn followed by a section in an extended conformation that replaces the helix and a long loop at the bottom of the molecule. The absence of any excursion on top of the molecule following beta-strands 5 and 6 results in a pronounced depression in the rim of the barrel. This depression forms one end of a shallow cleft that runs across the surface of the molecule, over the core of the beta-barrel to the area between the loops of units 1 and 2. The active site residues, Asp130 and Glu132, are located at the carboxyl end of beta-strand 4 and extend into this cleft. These residues are surrounded by several tyrosine residues. The cleft area formed by loops 1 and 2 is lined with polar residues, mainly asparagines. The latter area is thought to be responsible for oligosaccharide binding and recognition while the protein moiety of the substrate would be located outside the molecule but adjacent to the area of loops 5 and 6.(ABSTRACT TRUNCATED AT 250 WORDS)
Organizational Affiliation:
Wadsworth Center, New York State Department of Health, Albany 12201-0509.