Cooperative and Specific Binding of a RAMP Protein to Single-stranded CRISPR Repeat RNA
Wang, R., Zheng, H., Preamplume, G., Li, H.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Putative uncharacterized protein PH0350 | B [auth A], C [auth B] | 243 | Pyrococcus horikoshii | Mutation(s): 0 Gene Names: PH0350 | 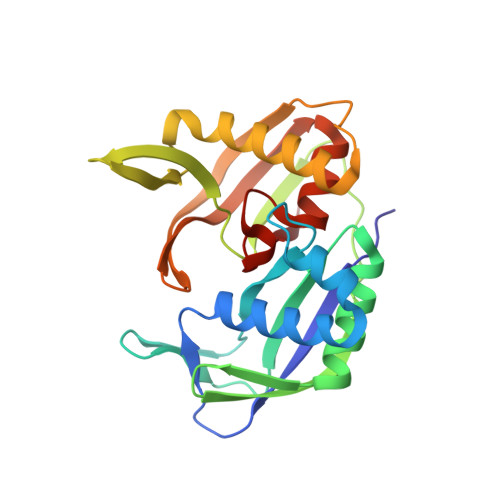 |
UniProt | |||||
Find proteins for O58088 (Pyrococcus horikoshii (strain ATCC 700860 / DSM 12428 / JCM 9974 / NBRC 100139 / OT-3)) Explore O58088 Go to UniProtKB: O58088 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O58088 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| RNA (5'-R(*GP*UP*UP*GP*AP*AP*AP*UP*CP*AP*GP*A)-3') | A [auth R], D [auth Q] | 12 | N/A | 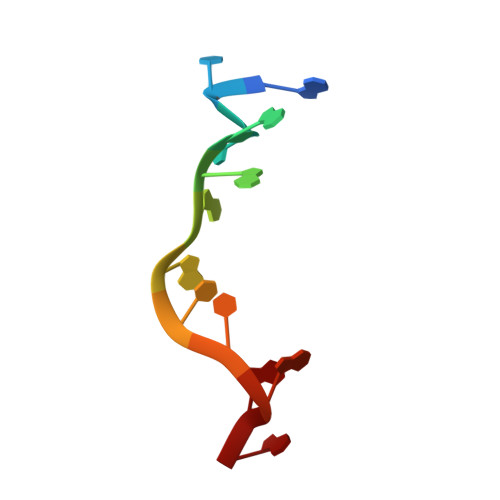 | |
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 75.269 | α = 90 |
| b = 75.269 | β = 90 |
| c = 257.351 | γ = 90 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| SOLVE | phasing |
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |