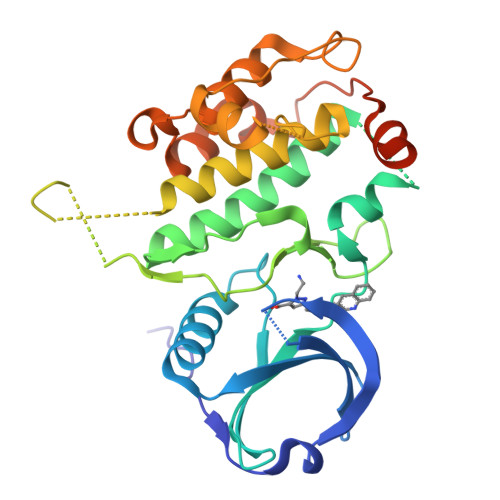
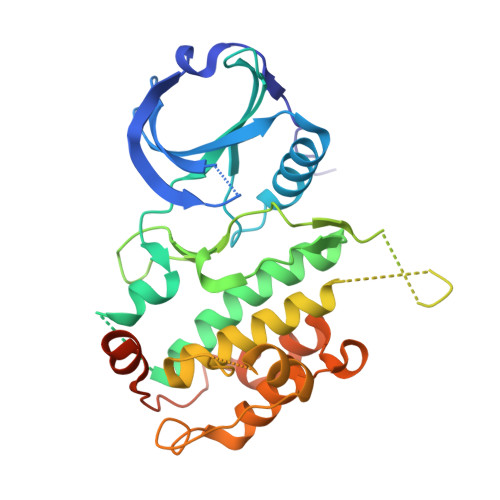
Structure-based lead identification of ATP-competitive MK2 inhibitors.
Barf, T., Kaptein, A., Wilde, S., Heijden, R., Someren, R., Demont, D., Schultz-Fademrecht, C., Versteegh, J., Zeeland, M., Seegers, N., Kazemier, B., Kar, B., Hoek, M., Roos, J., Klop, H., Smeets, R., Hofstra, C., Hornberg, J., Oubrie, A.(2011) Bioorg Med Chem Lett 21: 3818-3822
- PubMed: 21565500
- DOI: https://doi.org/10.1016/j.bmcl.2011.04.018
- Primary Citation of Related Structures:
3R1N, 3R2B, 3R2Y, 3R30 - PubMed Abstract:
MK2 kinase is a promising drug discovery target for the treatment of inflammatory diseases. Here, we describe the discovery of novel MK2 inhibitors using X-ray crystallography and structure-based drug design. The lead has in vivo efficacy in a short-term preclinical model.
Organizational Affiliation:
Merck Research Laboratories, MSD, Oss, The Netherlands.