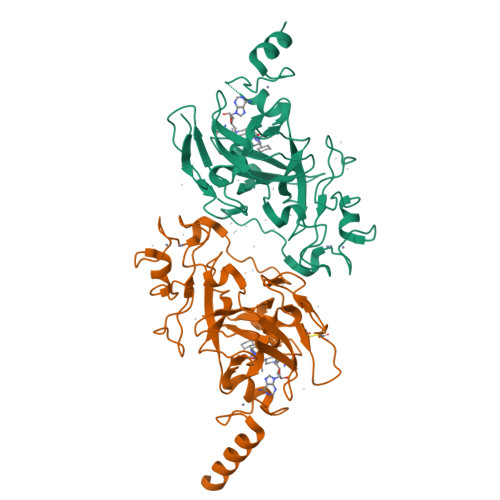
A chemical probe selectively inhibits G9a and GLP methyltransferase activity in cells.
Vedadi, M., Barsyte-Lovejoy, D., Liu, F., Rival-Gervier, S., Allali-Hassani, A., Labrie, V., Wigle, T.J., Dimaggio, P.A., Wasney, G.A., Siarheyeva, A., Dong, A., Tempel, W., Wang, S.C., Chen, X., Chau, I., Mangano, T.J., Huang, X.P., Simpson, C.D., Pattenden, S.G., Norris, J.L., Kireev, D.B., Tripathy, A., Edwards, A., Roth, B.L., Janzen, W.P., Garcia, B.A., Petronis, A., Ellis, J., Brown, P.J., Frye, S.V., Arrowsmith, C.H., Jin, J.(2011) Nat Chem Biol 7: 566-574
- PubMed: 21743462
- DOI: https://doi.org/10.1038/nchembio.599
- Primary Citation of Related Structures:
3RJW - PubMed Abstract:
Protein lysine methyltransferases G9a and GLP modulate the transcriptional repression of a variety of genes via dimethylation of Lys9 on histone H3 (H3K9me2) as well as dimethylation of non-histone targets. Here we report the discovery of UNC0638, an inhibitor of G9a and GLP with excellent potency and selectivity over a wide range of epigenetic and non-epigenetic targets. UNC0638 treatment of a variety of cell lines resulted in lower global H3K9me2 levels, equivalent to levels observed for small hairpin RNA knockdown of G9a and GLP with the functional potency of UNC0638 being well separated from its toxicity. UNC0638 markedly reduced the clonogenicity of MCF7 cells, reduced the abundance of H3K9me2 marks at promoters of known G9a-regulated endogenous genes and disproportionately affected several genomic loci encoding microRNAs. In mouse embryonic stem cells, UNC0638 reactivated G9a-silenced genes and a retroviral reporter gene in a concentration-dependent manner without promoting differentiation.
Organizational Affiliation:
Structural Genomics Consortium, University of Toronto, Toronto, Ontario, Canada.