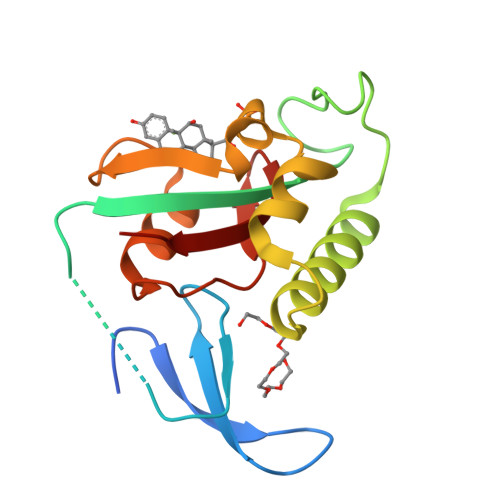
Selective targeting of disease-relevant protein binding domains by o-phosphorylated natural product derivatives.
Graber, M., Janczyk, W., Sperl, B., Elumalai, N., Kozany, C., Hausch, F., Holak, T.A., Berg, T.(2011) ACS Chem Biol 6: 1008-1014
- PubMed: 21797253
- DOI: https://doi.org/10.1021/cb2001796
- Primary Citation of Related Structures:
3TC5 - PubMed Abstract:
Phosphorylation-dependent protein binding domains are crucially important for intracellular signaling pathways and thus highly relevant targets in chemical biology. By screening of chemical libraries against 12 structurally diverse phosphorylation-dependent protein binding domains, we have identified fosfosal and dexamethasone-21-phosphate as selective inhibitors of two antitumor targets: the SH2 domain of the transcription factor STAT5b and the substrate-binding domain of the peptidyl-prolyl isomerase Pin1, respectively. Both compounds are phosphate prodrugs with documented clinical use as anti-inflammatory agents in humans and were discovered with a high hit rate from a small subgroup within the screening library. Our study indicates O-phosphorylation of appropriately preselected natural products or natural product derivatives as a generally applicable strategy for the identification of non-reactive and non-peptidic ligands of phosphorylation-dependent protein binding domains. Moreover, our data indicate that it would be advisable to monitor the bioactivities of clinically used prodrugs in their uncleaved state against phosphorylation-dependent protein binding domains.