Structure and Function of Copper Nitrite Reductase
Nojiri, M.(2016) Metalloenzymes In Denitrification: Applications And Environmental Impacts : 91-113
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2016) Metalloenzymes In Denitrification: Applications And Environmental Impacts : 91-113
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Copper-containing nitrite reductase | 336 | Achromobacter xylosoxidans | Mutation(s): 0 EC: 1.7.2.1 | 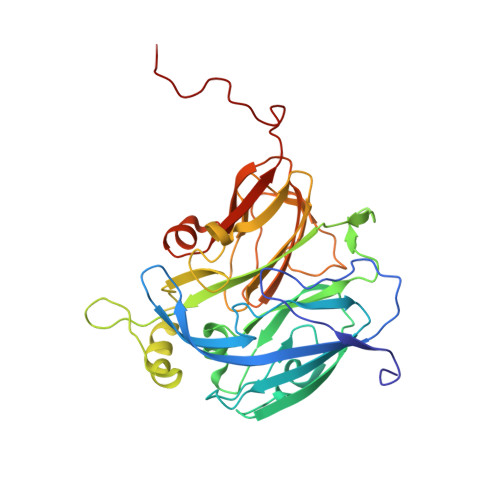 | |
UniProt | |||||
Find proteins for O68601 (Alcaligenes xylosoxydans xylosoxydans) Explore O68601 Go to UniProtKB: O68601 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O68601 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Blue copper protein | 124 | Hyphomicrobium denitrificans | Mutation(s): 0 | 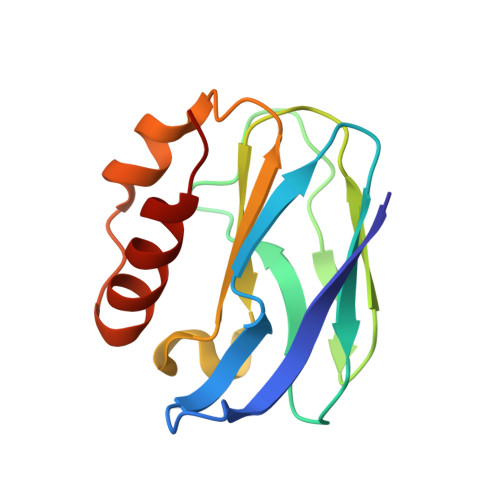 | |
UniProt | |||||
Find proteins for A7VL37 (Hyphomicrobium denitrificans) Explore A7VL37 Go to UniProtKB: A7VL37 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | A7VL37 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CU Query on CU | D [auth A], E [auth A], F [auth B], G [auth B], H [auth C] | COPPER (II) ION Cu JPVYNHNXODAKFH-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 153.226 | α = 90 |
| b = 153.226 | β = 90 |
| c = 153.226 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-2000 | data reduction |
| SCALEPACK | data scaling |
| MOLREP | phasing |