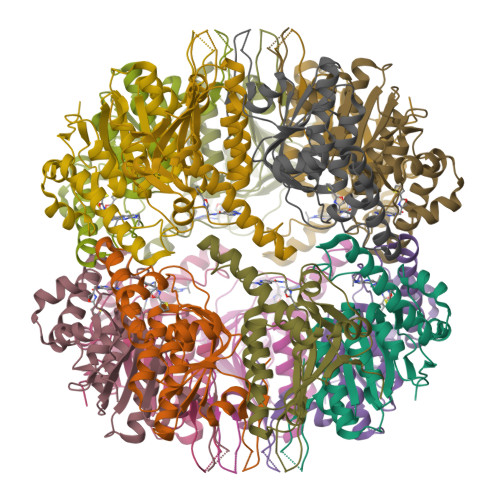
Reversible Inhibitors Arrest ClpP in a Defined Conformational State that Can Be Revoked by ClpX Association.
Pahl, A., Lakemeyer, M., Vielberg, M.T., Hackl, M.W., Vomacka, J., Korotkov, V.S., Stein, M.L., Fetzer, C., Lorenz-Baath, K., Richter, K., Waldmann, H., Groll, M., Sieber, S.A.(2015) Angew Chem Int Ed Engl 54: 15892-15896
- PubMed: 26566002
- DOI: https://doi.org/10.1002/anie.201507266
- Primary Citation of Related Structures:
5DL1 - PubMed Abstract:
Caseinolytic protease P (ClpP) is an important regulator of Staphylococcus aureus pathogenesis. A high-throughput screening for inhibitors of ClpP peptidase activity led to the identification of the first non-covalent binder for this enzyme class. Co-crystallization of the small molecule with S. aureus ClpP revealed a novel binding mode: Because of the rotation of the conserved residue proline 125, ClpP is locked in a defined conformational state, which results in distortion of the catalytic triad and inhibition of the peptidase activity. Based on these structural insights, the molecule was optimized by rational design and virtual screening, resulting in derivatives exceeding the potency of previous ClpP inhibitors. Strikingly, the conformational lock is overturned by binding of ClpX, an associated chaperone that enables proteolysis by substrate unfolding in the ClpXP complex. Thus, regulation of inhibitor binding by associated chaperones is an unexpected mechanism important for ClpP drug development.
Organizational Affiliation:
Center for Integrated Protein Science at the Department of Chemistry, Technische Universität München, Lichtenbergstrasse 4, 85747 Garching (Germany).