New imine-reducing enzymes from beta-hydroxyacid dehydrogenases by single amino acid substitutions.
Lenz, M., Fademrecht, S., Sharma, M., Pleiss, J., Grogan, G., Nestl, B.M.(2018) Protein Eng Des Sel 31: 109-120
- PubMed: 29733377
- DOI: https://doi.org/10.1093/protein/gzy006
- Primary Citation of Related Structures:
5OCM - PubMed Abstract:
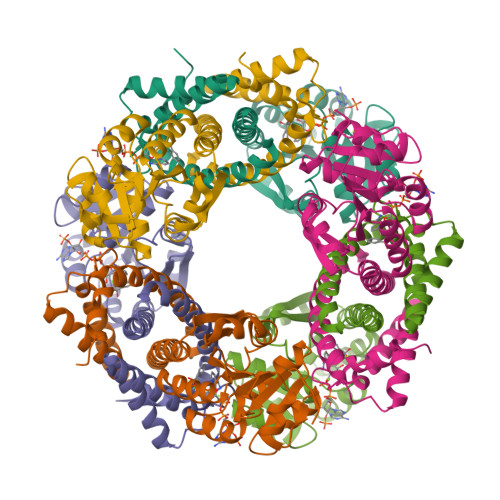
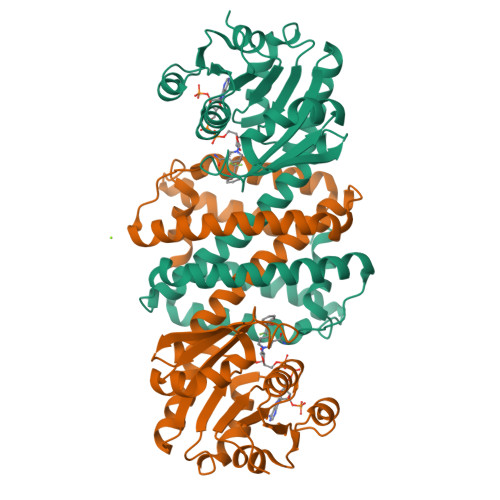
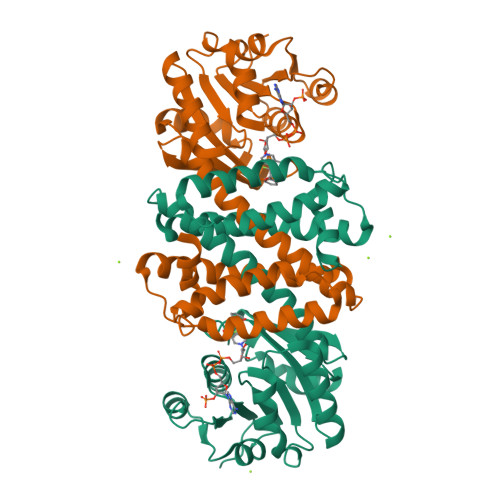
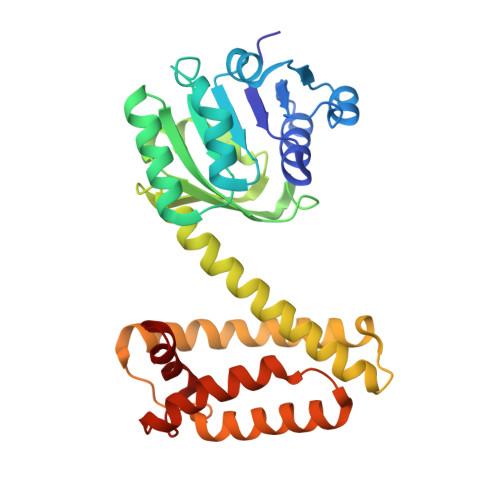
We report the exploration of the evolutionary relationship between imine reductases (IREDs) and other dehydrogenases. This approach is informed by the sequence similarity between these enzyme families and the recently described promiscuous activity of IREDs for the highly reactive carbonyl compound 2,2,2-trifluoroacetophenone. Using the structure of the R-selective IRED from Streptosporangium roseum (R-IRED-Sr) as a model, β-hydroxyacid dehydrogenases (βHADs) were identified as the dehydrogenases most similar to IREDs. To understand how active site differences in IREDs and βHADs enable the reduction of predominantly C = N or C = O bonds respectively, we substituted amino acid residues in βHADs with the corresponding residues from the R-IRED-Sr and were able to increase the promiscuous activity of βHADs for C = N functions by a single amino acid substitution. Variants βHADAt_K170D and βHADAt_K170F lost mainly their keto acid reduction activity and gained the ability to catalyze the reduction of imines. Moreover, the product enantiomeric purity for a bulky imine substrate could be increased from 23% ee (R-IRED-Sr) to 97% ee (βHADAt_K170D/F_F231A) outcompeting already described IRED selectivity.
Organizational Affiliation:
Institute of Biochemistry and Technical Biochemistry, Department of Chemistry, Universitaet Stuttgart, Allmandring 31, Stuttgart, Germany.