Structural Insights into Thioether Bond Formation in the Biosynthesis of Sactipeptides.
Grove, T.L., Himes, P.M., Hwang, S., Yumerefendi, H., Bonanno, J.B., Kuhlman, B., Almo, S.C., Bowers, A.A.(2017) J Am Chem Soc 139: 11734-11744
- PubMed: 28704043
- DOI: https://doi.org/10.1021/jacs.7b01283
- Primary Citation of Related Structures:
5WGG, 5WHY - PubMed Abstract:
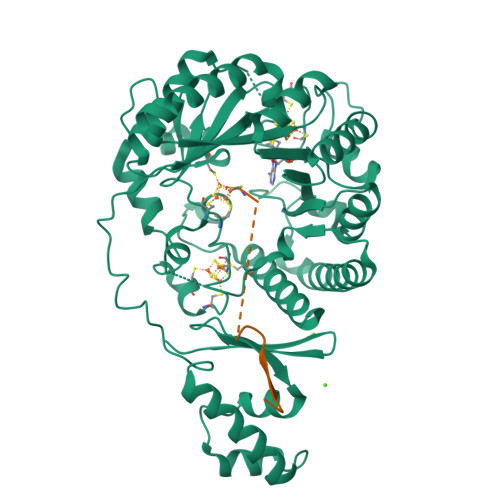
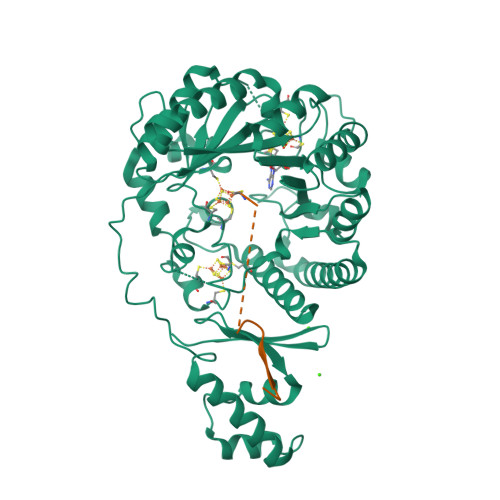
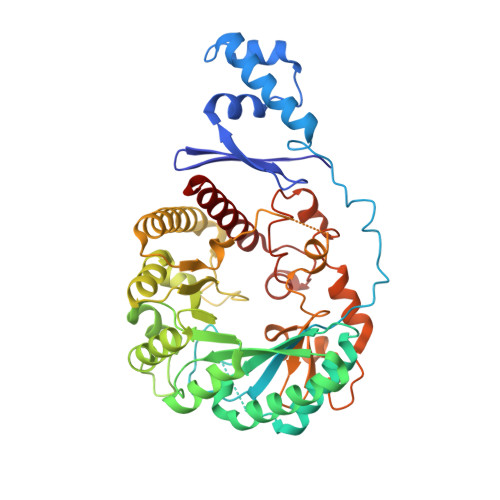
Sactipeptides are ribosomally synthesized peptides that contain a characteristic thioether bridge (sactionine bond) that is installed posttranslationally and is absolutely required for their antibiotic activity. Sactipeptide biosynthesis requires a unique family of radical SAM enzymes, which contain multiple [4Fe-4S] clusters, to form the requisite thioether bridge between a cysteine and the α-carbon of an opposing amino acid through radical-based chemistry. Here we present the structure of the sactionine bond-forming enzyme CteB, from Clostridium thermocellum ATCC 27405, with both SAM and an N-terminal fragment of its peptidyl-substrate at 2.04 Å resolution. CteB has the (β/α) 6 -TIM barrel fold that is characteristic of radical SAM enzymes, as well as a C-terminal SPASM domain that contains two auxiliary [4Fe-4S] clusters. Importantly, one [4Fe-4S] cluster in the SPASM domain exhibits an open coordination site in absence of peptide substrate, which is coordinated by a peptidyl-cysteine residue in the bound state. The crystal structure of CteB also reveals an accessory N-terminal domain that has high structural similarity to a recently discovered motif present in several enzymes that act on ribosomally synthesized and post-translationally modified peptides (RiPPs), known as a RiPP precursor peptide recognition element (RRE). This crystal structure is the first of a sactionine bond forming enzyme and sheds light on structures and mechanisms of other members of this class such as AlbA or ThnB.
Organizational Affiliation:
Department of Biochemistry, Albert Einstein College of Medicine , Bronx, New York 10461, United States.