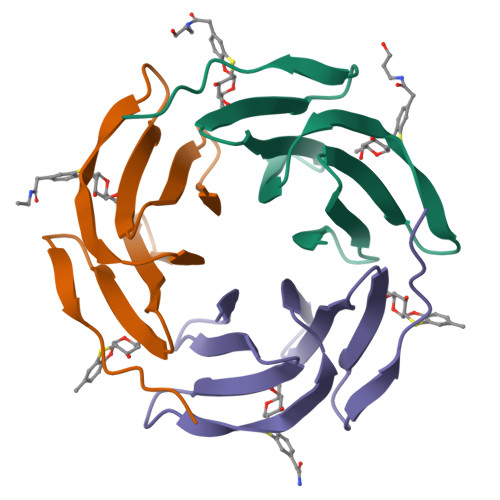
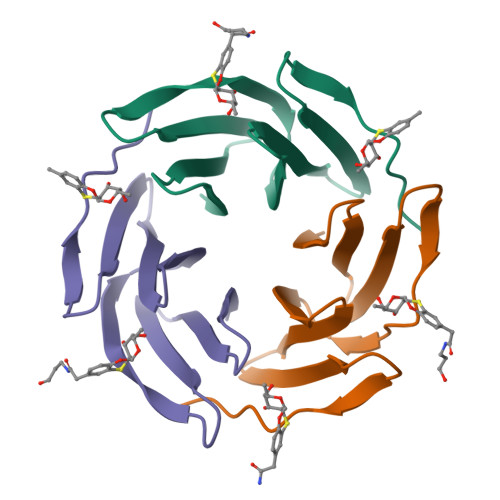
Fucosylated ubiquitin and orthogonally glycosylated mutant A28C: conceptually new ligands for Burkholderia ambifaria lectin (BambL).
Kuhaudomlarp, S., Cerofolini, L., Santarsia, S., Gillon, E., Fallarini, S., Lombardi, G., Denis, M., Giuntini, S., Valori, C., Fragai, M., Imberty, A., Dondoni, A., Nativi, C.(2020) Chem Sci 11: 12662-12670
- PubMed: 34094460
- DOI: https://doi.org/10.1039/d0sc03741a
- Primary Citation of Related Structures:
6ZFC - PubMed Abstract:
Two orthogonal, metal free click reactions, enabled to glycosylate ubiquitin and its mutant A28C forming two protein scaffolds with high affinity for BambL, a lectin from the human pathogen Burkholderia ambifaria . A new fucoside analogue, with high affinity with BambL, firstly synthetized and co-crystallized with the protein target, provided the insights for sugar determinants grafting onto ubiquitin. Three ubiquitin-based glycosides were thus assembled. Fuc-Ub , presented several copies of the fucoside analogue, with proper geometry for multivalent effect; Rha-A28C , displayed one thio-rhamnose, known for its ability to tuning the immunological response; finally, Fuc-Rha-A28C , included both multiple fucoside analogs and the rhamnose residue. Fuc-Ub and Fuc-Rha-A28C ligands proved high affinity for BambL and unprecedented immune modulatory properties towards macrophages activation.
Organizational Affiliation:
Université Grenoble Alpes, CNRS, CERMAV 38000 Grenoble France.