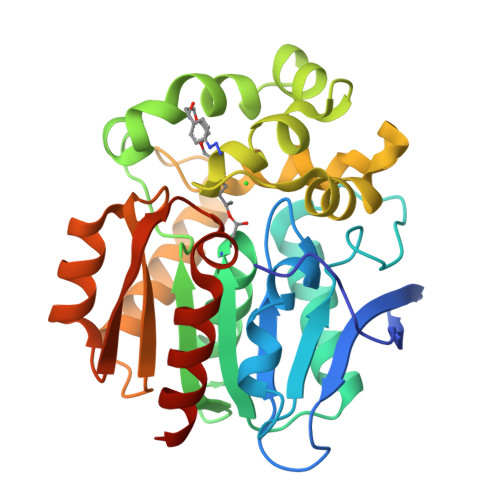
A chemical tool for blue light-inducible proximity photo-crosslinking in live cells.
Mishra, P.K., Kang, M.G., Lee, H., Kim, S., Choi, S., Sharma, N., Park, C.M., Ko, J., Lee, C., Seo, J.K., Rhee, H.W.(2022) Chem Sci 13: 955-966
- PubMed: 35211260
- DOI: https://doi.org/10.1039/d1sc04871f
- Primary Citation of Related Structures:
7WAM, 7WAN - PubMed Abstract:
We developed a proximity photo-crosslinking method ( Spotlight ) with a 4-azido- N -ethyl-1,8-naphthalimide (AzNP) moiety that can be converted to reactive aryl nitrene species using ambient blue light-emitting diode light. Using an AzNP-conjugated HaloTag ligand (VL1), blue light-induced photo-crosslinked products of various HaloTag-conjugated proteins of interest were detected in subcellular spaces in live cells. Chemical or heat stress-induced dynamic changes in the proteome were also detected, and photo-crosslinking in the mouse brain tissue was enabled. Using Spotlight , we further identified the host interactome of SARS-CoV-2 nucleocapsid (N) protein, which is essential for viral genome assembly. Mass analysis of the VL1-crosslinked product of N -HaloTag in HEK293T cells showed that RNA-binding proteins in stress granules were exclusively enriched in the cross-linked samples. These results tell that our method can reveal the interactome of protein of interest within a short distance in live cells.
Organizational Affiliation:
Department of Chemistry, Seoul National University Seoul 08826 Korea rheehw@snu.ac.kr.