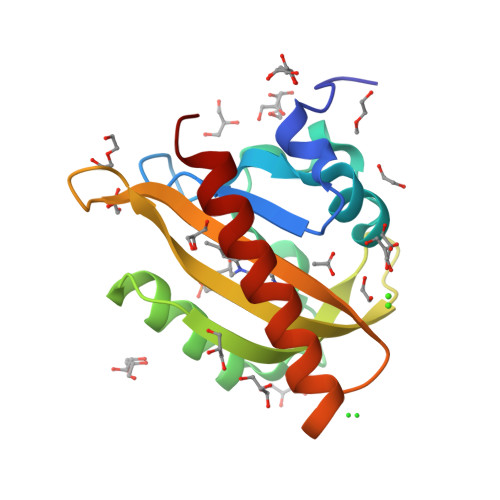
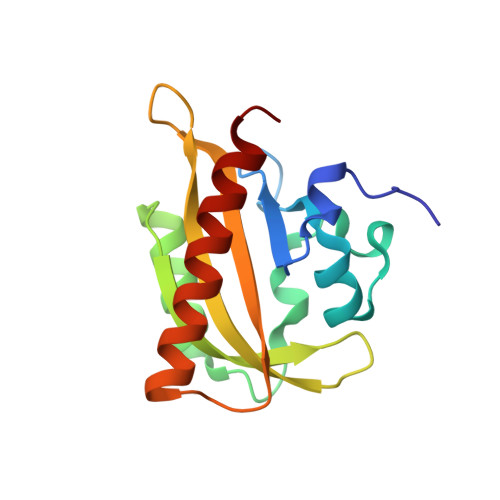
Signal transduction in light-oxygen-voltage receptors lacking the active-site glutamine.
Dietler, J., Gelfert, R., Kaiser, J., Borin, V., Renzl, C., Pilsl, S., Ranzani, A.T., Garcia de Fuentes, A., Gleichmann, T., Diensthuber, R.P., Weyand, M., Mayer, G., Schapiro, I., Moglich, A.(2022) Nat Commun 13: 2618-2618
- PubMed: 35552382
- DOI: https://doi.org/10.1038/s41467-022-30252-4
- Primary Citation of Related Structures:
7PGX, 7PGY, 7PGZ, 7PH0 - PubMed Abstract:
In nature as in biotechnology, light-oxygen-voltage photoreceptors perceive blue light to elicit spatiotemporally defined cellular responses. Photon absorption drives thioadduct formation between a conserved cysteine and the flavin chromophore. An equally conserved, proximal glutamine processes the resultant flavin protonation into downstream hydrogen-bond rearrangements. Here, we report that this glutamine, long deemed essential, is generally dispensable. In its absence, several light-oxygen-voltage receptors invariably retained productive, if often attenuated, signaling responses. Structures of a light-oxygen-voltage paradigm at around 1 Å resolution revealed highly similar light-induced conformational changes, irrespective of whether the glutamine is present. Naturally occurring, glutamine-deficient light-oxygen-voltage receptors likely serve as bona fide photoreceptors, as we showcase for a diguanylate cyclase. We propose that without the glutamine, water molecules transiently approach the chromophore and thus propagate flavin protonation downstream. Signaling without glutamine appears intrinsic to light-oxygen-voltage receptors, which pertains to biotechnological applications and suggests evolutionary descendance from redox-active flavoproteins.
Organizational Affiliation:
Department of Biochemistry, University of Bayreuth, 95447, Bayreuth, Germany.