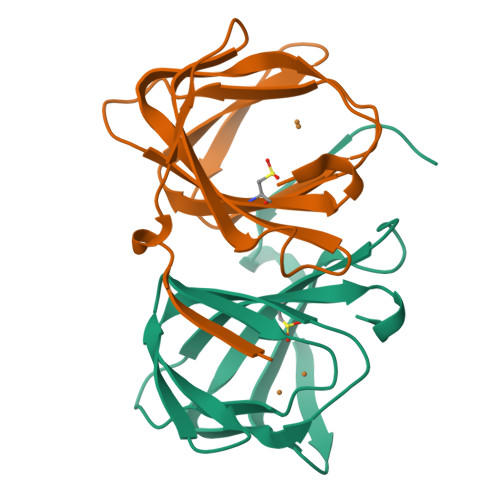
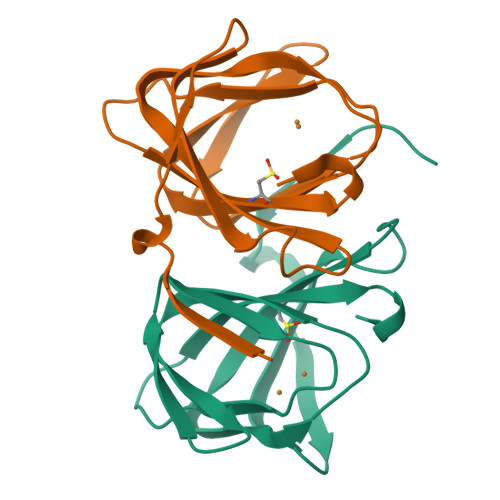
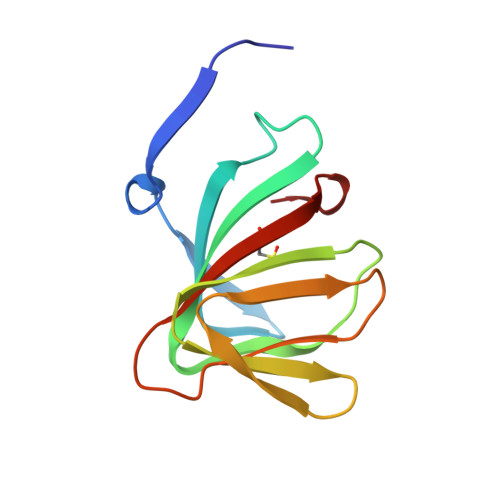
An artificial metallolyase with pliable 2-His-1-carboxylate facial triad for stereoselective Michael addition.
Matsumoto, R., Yoshioka, S., Yuasa, M., Morita, Y., Kurisu, G., Fujieda, N.(2023) Chem Sci 14: 3932-3937
- PubMed: 37035687
- DOI: https://doi.org/10.1039/d2sc06809e
- Primary Citation of Related Structures:
8HJX, 8HJY, 8HJZ - PubMed Abstract:
We repurposed the metal-binding site of a cupin superfamily protein into the 2-His-1-carboxylate facial triad, which is one of the common motifs in natural non-heme enzymes, to construct artificial metalloenzymes that can catalyze new-to-nature reactions. The Cu 2+ -H52A/H58E variant catalyzed the stereoselective Michael addition reaction and was found to bear a flexible metal-binding site in the high-resolution crystal structure. Furthermore, the H52A/H58E/F104W mutant accommodated a water molecule, which was supported by Glu58 and Trp104 residues via hydrogen bonding, presumably leading to high stereoselectivity. Thus, the 2-His-1-carboxylate facial triad was confirmed to be a versatile and promising metal-binding motif for abiological and canonical biological reactions.
Organizational Affiliation:
Department of Applied Biological Chemistry, Graduate School of Agriculture, Osaka Metropolitan University 1-1 Gakuen-cho, Naka-ku Sakai-shi Osaka 599-8531 Japan fujieda@omu.ac.jp.