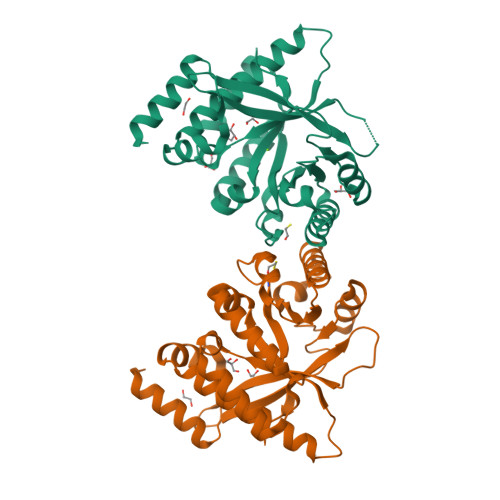
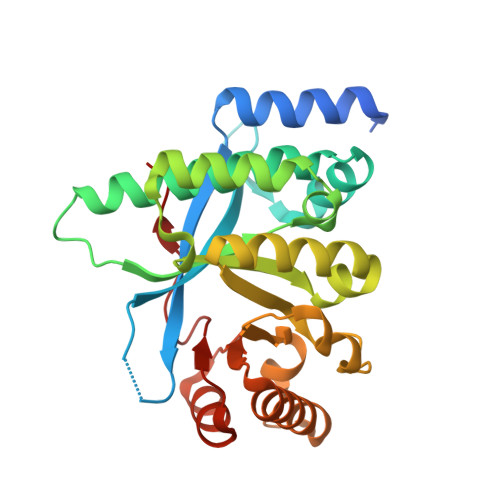
Control of phosphodiesterase activity in the regulator of biofilm dispersal RbdA from Pseudomonas aeruginosa.
Cordery, C., Craddock, J., Maly, M., Basavaraja, K., Webb, J.S., Walsh, M.A., Tews, I.(2024) RSC Chem Biol 5: 1052-1059
- PubMed: 39247681
- DOI: https://doi.org/10.1039/d4cb00113c
- Primary Citation of Related Structures:
8PPS - PubMed Abstract:
The switch between planktonic and biofilm lifestyle correlates with intracellular concentration of the second messenger bis-(3'-5')-cyclic dimeric guanosine monophosphate (c-di-GMP). While bacteria possess cyclase and phosphodiesterase enzymes to catalyse formation or hydrolysis of c-di-GMP, both enzymatic domains often occur in a single protein. It is tacitly assumed that one of the two enzymatic activities is dominant, and that additional domains and protein interactions enable responses to environmental conditions and control activity. Here we report the structure of the phosphodiesterase domain of the membrane protein RbdA (regulator of biofilm dispersal) in a dimeric, activated state and show that phosphodiesterase activity is controlled by the linked cyclase. The phosphodiesterase region around helices α5/α6 forms the dimer interface, providing a rationale for activation, as this region was seen in contact with the cyclase domain in an auto-inhibited structure previously described. Kinetic analysis supports this model, as the activity of the phosphodiesterase alone is lower when linked to the cyclase. Analysis of a computed model of the RbdA periplasmatic domain reveals an all-helical architecture with a large binding pocket that could accommodate putative ligands. Unravelling the regulatory circuits in multi-domain phosphodiesterases like RbdA is important to develop strategies to manipulate or disperse bacterial biofilms.
Organizational Affiliation:
Biological Sciences, Institute for Life Sciences, University of Southampton Southampton SO17 1BJ UK ivo.tews@soton.ac.uk.